Cathodic protection of offshore structures
Offshore structures
Offshore structures include platforms, semi-submerged structures in water or wells, pipelines, and underwater equipment. Offshore steel structures are designed based on two criteria of strength coefficient and strength. Its strength coefficient depends on the amount of surface material that is lost during the corrosion process. Its strength is affected by pitting corrosion. Therefore, a proper and accurate design of a corrosion control system is essential.
The outer area of offshore fixed steel structures is divided into two categories according to its surroundings.
The part of the structure that is adjacent to the air and above the water surface and must be protected by a suitable coating. Another part that is under water and to protect it, a precise cathodic protection system is required.
Cathodic protection system
When the structure is inside the electrolyte (water), the potential difference between the different points of the structure causes a current to flow between these points. This current causes the part that has a more negative voltage to corrode. The principle of cathodic protection by creating a more negative potential around the structure leads to the potential of different parts of the structure being the same. Therefore, the steel structure is protected against corrosion. This current may be supplied by various sources such as transformer rectifiers or sacrificial anodes.
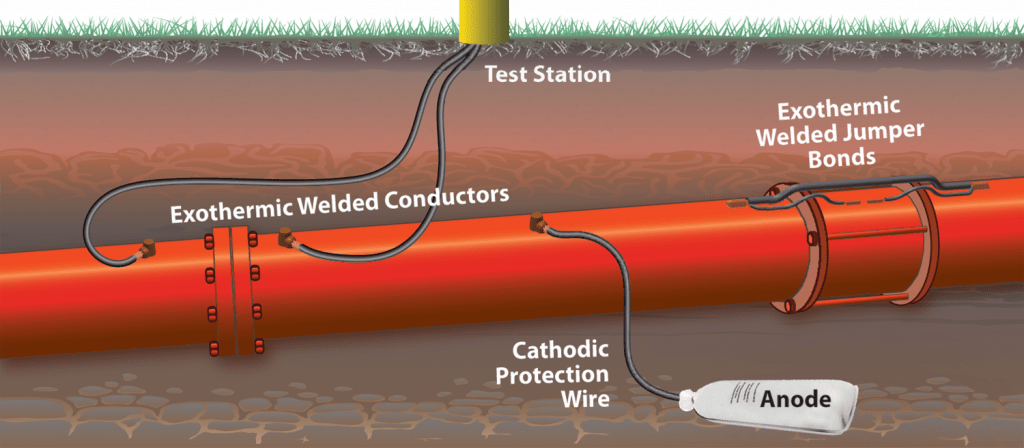
Cathodic protection by sacrificial anode
The sacrificial anode is usually a metal that has a more negative potential than the structure. The connection of the anode to the structure causes a current to flow inside the electrolyte from the anode to the structure. For offshore structures, the sacrificial anodes are made of aluminum or zinc. Aluminum sacrificial anode is used more than zinc sacrificial anode due to its “better current to weight ratio” feature.
Types of sacrificial aluminum anodes widely used in offshore structures
Important types of sacrificial aluminum anodes designed for offshore applications include bracelet anode, stand-off anode, and welding anode.
Core anodes are connected to the bases by means of welds or bolts before installing the structure in the sea. These anodes are generally installed on offshore jackets.

Aluminum bracelet anodes are specially designed for cathodic protection of oil and gas pipelines. These anodes are produced from alloys with high purity and very low percentage of iron and are designed with diameters appropriate to the desired pipe diameter. These anodes usually consist of two parts that are connected to each other by an internal iron belt.

- Published in Articles, Educational, Scientific
Cathodic protection of pipelines
The role of cathodic protection of pipelines in the oil and gas industry
One of the main and very costly problems in large industries such as gas, oil, petrochemical, shipping, etc., is the corrosion of metal structures.
In the oil and gas industry, carbon steel pipelines are used to transport hydrocarbons from jackets to production platforms and storage tanks, and enter refineries through pipelines. Carbon steel pipelines are the safest and least expensive way to transport large volumes of hydrocarbons over long distances.
Fuel and water transmission pipelines and other structures buried in soil or submerged in water, due to their metallurgical conditions, are in a state of corrosion and after a while, the performance of the structure is disrupted.
Corrosion in the oil and gas industry dramatically increases the overhead costs of plumbing operations due to the replacement of worn equipment and damage to adjacent equipment. Therefore, it is necessary to use a proper protection system to prevent corrosion.
Corrosion control mechanism in cathodic protection of pipelines
Corrosion control mechanisms in oil and gas pipelines are mitigation measures to eliminate or reduce corrosion to prevent pipeline failure and related consequences such as crop failure, environmental pollution and potential accidents.
Five control mechanisms to minimize pipeline corrosion and cathodic protection of pipelines that must be considered during the design and construction of pipelines include design, material selection, coating, cathodic protection, and chemical protection.
Designing
One of the important factors in corrosion control is design. Proper design of a steel pipe increases the life of the structure. Gaps, steel geometry, sharp edges, welded joints, and pipeline connections such as flanges, valves, and pipe supports are often subject to local corrosion due to fluid penetration and dissolution.
In addition, these environments have poor coverage due to inaccessibility or sensitivity to coverage failure. These factors contribute to corrosion and should be carefully considered during the design phase to limit corrosion.
Material selection
The choice of materials is very basic in terms of engineering design to reduce corrosion. The choice of material depends on the environmental conditions such as the fluid being transferred, the temperature, the pressure and the electrolyte in which the pipe is placed (water or soil). Also in flange joints, piping and general joints where it is possible to pair metals of different materials, to prevent galvanic corrosion, care must be taken in determining the material.
Due to its good mechanical properties, availability and low cost, carbon steel, despite its low corrosion resistance compared to other corrosion-resistant alloys, is the main material for pipeline transmission.
coating
One of the main and simplest methods of protecting pipelines is coating. A suitable coating prevents direct contact of the outer surface of the tube with the surrounding environment and thus prevents the flow of ions through the electrolyte, thus limiting corrosion.
There are different methods and types of coating and the choice of its type depends on the type of material selected:
- Painting
Painting is used in environments where the equipment is only exposed to air and no electrolyte is available.
- Coating
The main types of coatings for coating structures that are exposed to electrolytes are strong coatings such as polyethylenes, bitumen and polypropylene.
- Lining
Lining the surface of the structure with rubber sheets.
- Cladding
coating one metal with another.
- Cathodic Protection
Using a cathodic protection system along with coating is an effective way to prevent corrosion of pipelines.
There are two general methods for cathodic protection of pipelines:
- Sacrificial anode system
- Impressed current anode system
In cases where the length of the pipeline is very long and in the presence of stray currents, an impressed current system is used to protect the pipeline.
Chemical protection
Chemical protection includes corrosion inhibitors, Oxygen purification system, and biocide in a chemical process.
In the chemical process, biocide is injected into the system, liquid or water to kill microorganisms such as reduced bacteria that increase corrosion under microbiological effects with sulfide. Oxygen scavenging chemicals are injected into the water to deoxygenate to reduce the severity of the corrosion and ultimately achieve adequate cathodic protection of the pipelines.
- Published in Articles, Educational, Scientific
Cathodic protection of underground storage tanks
Corrosion of underground storage tanks
Underground storage tanks are generally in contact with materials such as soil and sand. The electrochemical reaction between the tank and the surrounding soil (electrolyte) leads to tank corrosion. The small voltage difference on the steel surface causes a current to flow from one part of the tank to another. Corrosion occurs where current flows into the soil (electrolyte). This part in the galvanic cell is called the anode. Where the flow from the soil enters the reservoir is the cathode and corrosion does not occur.
Prevention of tank corrosion (cathodic protection of underground storage tanks)
An outer cover as well as a cathodic protection system are used to protect the underground tanks from corrosion. A suitable outer cover can protect more than 99% of the tank surface. But since there is no complete cover, in addition to the outer cover, a cathodic protection system must be used to protect the tank from corrosion. The cathodic protection system converts the tank into a cathode by applying an external current and protects it from corrosion. In cases where the amount of current required to protect the structure is small, sacrificial anodes are used. For large structures such as large diameter pipelines, an impressed current system is used.
Magnesium sacrificial anode for cathodic protection of underground storage tanks
Magnesium sacrificial anode is the most common sacrificial anode used in cathodic protection of underground tanks. Magnesium anode can protect underground tanks in any soil. To reduce the electrical resistance of the anode to soil, a magnesium anode is usually used inside a linen bag with backfill material that is a combination of gypsum plaster, bentonite and sodium sulfate. Magnesium anode is produced in two types of standard and high potential. High potential magnesium anode is used in soils with a resistance of about 10,000 ohm-cm. Based on the dimensions of the tank and the soil resistance in which the tank is located, the number and dimensions of the required anodes are determined. The two most common sizes of magnesium anodes to protect underground reservoirs are 17-pound and 32-pound anodes. The anodes are installed at a suitable distance and depth from the tank. The anode wire is connected to the top of the tank with a low-resistance electrical connection. All connections are covered with waterproofing.

In terms of cathodic protection of underground storage tanks, the technical and engineering department of Tavanazob Sana’ati Kavir Company is ready to provide consulting services for which you can contact us.
- Published in Articles, Educational, Scientific
What is an impressed current cathodic protection system?
What is a impressed current cathodic protection system and how does it work?
For cathodic protection in large structures, the use of cathodic protection system of sacrificial anodes may not be appropriate. The number of sacrificial anodes required to provide sufficient current to protect the structure against corrosion may be excessive or impractical. To control this, an external energy source is used to help conduct electrochemical reactions. are Impressed current cathodic protection system is a type of system that is usually used in cases where high flow requirements required to protect against corrosion or control needs to be increased. In impressed current cathode protection (ICCP) systems, the current required for protection is supplied from an external source of electrical energy supplied by a DC regulated power supply called a transformer. ICCP systems for corrosion protection are ideal for many structures, such as underground pipelines, storage tanks, large ships, and offshore and offshore structures.

How does a impressed current cathodic protection system work?
The impressed current cathodic protection system consists of one or more reference electrodes and several ICCP anodes, all connected to the same power supply. The reference electrodes adjust the required output current unit on the anodes by measuring the electrical protection potential of the environment in which the structure is located. Adjusted current prevents the corrosion process from occurring. In this method, unlike the sacrificial anode method, where the anodes were negative, the anodes are more positive than the structure. The positive pole of the power supply is connected to the anode and the negative pole is connected to the structure. Therefore, the positive ions inside the electrolyte return from the anode to the metal in question. Depending on the electrolyte in which the structure is located, the applied voltage must be such that it can withstand sufficient current intensity for all parts of the device that is cathodically protected. Provide. A properly installed, commissioned, and serviced ICCP system can last for 25 years or more.
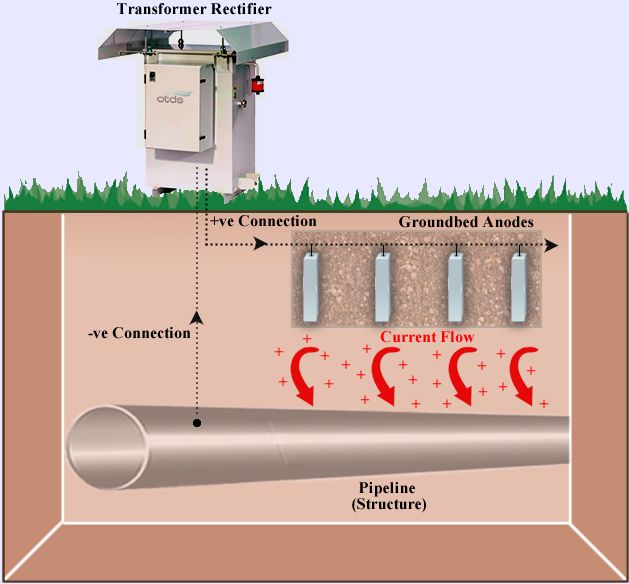
impressed current anodes
The cathodic protection system consists of two types of anodes: the sacrificial anode and the impressed current anode.
A impressed current anode uses an external power source known as a “rectifier” to create a high potential difference between the protected surface and the anode. The anodes used are generally made of graphite, cast iron, titanium alloy and silicon iron. Today, the most common type of impressed current anodes are MMO anodes, which are found in various sizes and shapes such as wires, rods, tubes, plates and strips. The choice of the type of impressed current anodes to protect the structure from corrosion depends on the environment in which the structure is located. For example, ribbon MMO anodes are used to protect the bottom of tanks.
Impressed current anodes have advantages over sacrificial anodes, including:
- They have higher flow efficiencies.
- Less anodes are needed to protect the structure.
- Reduce the initial cost.
- Published in Articles, Educational, Scientific
Types of sacrificial anode system backfills and its applications
Types of backfill and its applications:
In cathodic protection systems of pipelines or underground structures buried in the soil, the anode used is not in direct contact with the soil. This is because the minerals and chemicals in the soil may interfere with the conductivity of the stream by creating a high-strength layer. To increase the efficiency of the anode, sometimes magnesium and zinc sacrificial anodes are provided in linen bags with a backfill. The backfill material quickly absorbs soil moisture and reduces electrolyte resistance, thus improving the practical implementation of protection. Backfill materials generally include gypsum plaster, bentonite and sodium sulfate. Depending on the environment in which the anode is placed, one of the types of backfill, which is considered in the table below, is used.
Table of combinations of different types of backfills according to IPS-M-TP-750 standard:
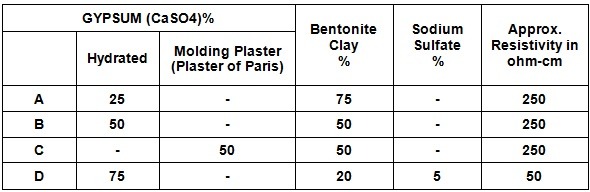
Due to its moisture retention properties, bentonite type A is used in areas where soil moisture is low.
Type B backfill is commonly used for sacrificial zinc anodes.
Type C backfill is useful for magnesium or zinc sacrificial anode in very wet or swampy soils to prevent the backfill from leaving the anode surface quickly.
Type D backfill, this type of backfill has low resistance and is used in areas where soil resistance is high to reduce the resistance of the anode to ground.
Dimensions of backfill bag
According to IPS-M-TP-750 standard, the diameter of the backfill bag must be at least 50 mm larger than the diameter of the anode. The backfill material should be tightly packed in the bag to prevent the anode from moving and to get enough coverage around the anode.

- Published in Articles, Educational, Scientific
What is a sacrificial anode?
How does a sacrificial anode protect metal structures?
Corrosion of metal structures immersed in water or buried in soil will cause very dangerous and costly damage. One way to protect these structures is to use sacrificial anodes. The sacrificial anode sacrifices itself due to the electrochemical process of corrosion by destroying instead of the impeller, impeller branch, motor or other metal components in water or soil.
Sacrificial anodes are relatively inexpensive metal parts. Known as active metals, they are specifically designed to corrode structures instead of expensive metal parts. These anodes are made of metals that have a more negative voltage than the structural metal. Sacrificial anodes were usually made of zinc metal, hence they are also called “zinc anodes“, but magnesium or aluminum alloy can also be used to produce them. Each of these metals has different properties and applications. The electrical potential and flow capacity of anodic metals are two important features in determining the type and extent of sacrificial anodes used. Sacrificial anodes come in all shapes and sizes, but they all work the same way. They are electrically attached to the metal structure to protect it from corrosion.
How does the sacrificial anode work?
The potential difference between the sacrificial anode and the protected structure causes a current in the electrolyte to flow from the anode to the cathode. The anode and cathode must both be inside an electrolyte and have an electrical connection between them. The sacrificial anode is a metal that has a more negative potential than the structure and forms the negative pole of a corrosion cell. The positive pole of this corrosion cell is the metal (cathode) that protects it. The saltier or more polluted the water, the faster the sacrificial anode will erode.
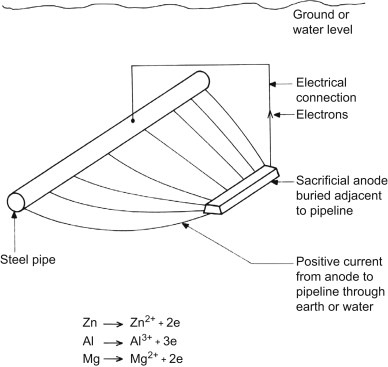
Since the output current of the sacrificial anodes is much lower than that of the impressed current anodes, these anodes are used in places where a small current is required for protection. Also, in places such as submarine pipelines where we do not have access to electricity and current regulation, the sacrificial anode system is used.
- Published in Articles, Educational, Scientific








