What is a sacrificial anode?
How does a sacrificial anode protect metal structures?
Corrosion of metal structures immersed in water or buried in soil will cause very dangerous and costly damage. One way to protect these structures is to use sacrificial anodes. The sacrificial anode sacrifices itself due to the electrochemical process of corrosion by destroying instead of the impeller, impeller branch, motor or other metal components in water or soil.
Sacrificial anodes are relatively inexpensive metal parts. Known as active metals, they are specifically designed to corrode structures instead of expensive metal parts. These anodes are made of metals that have a more negative voltage than the structural metal. Sacrificial anodes were usually made of zinc metal, hence they are also called “zinc anodes“, but magnesium or aluminum alloy can also be used to produce them. Each of these metals has different properties and applications. The electrical potential and flow capacity of anodic metals are two important features in determining the type and extent of sacrificial anodes used. Sacrificial anodes come in all shapes and sizes, but they all work the same way. They are electrically attached to the metal structure to protect it from corrosion.
How does the sacrificial anode work?
The potential difference between the sacrificial anode and the protected structure causes a current in the electrolyte to flow from the anode to the cathode. The anode and cathode must both be inside an electrolyte and have an electrical connection between them. The sacrificial anode is a metal that has a more negative potential than the structure and forms the negative pole of a corrosion cell. The positive pole of this corrosion cell is the metal (cathode) that protects it. The saltier or more polluted the water, the faster the sacrificial anode will erode.
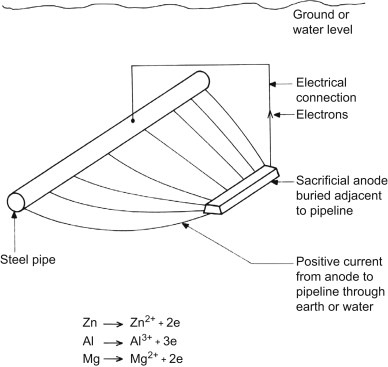
Since the output current of the sacrificial anodes is much lower than that of the impressed current anodes, these anodes are used in places where a small current is required for protection. Also, in places such as submarine pipelines where we do not have access to electricity and current regulation, the sacrificial anode system is used.
- Published in Articles, Educational, Scientific
What is the cathodic protection?
Cathodic protection and its different types
Cathodic protection is a way to reduce corrosion by minimizing the potential difference between the anode and the cathode. This is achieved by creating a stream from an external source on the structure to be protected (such as a pipeline). When enough current is used, the whole structure will be at a potential. Therefore, there will be no anode and cathode sites. Cathodic protection is commonly used to protect numerous structures against corrosion, such as ships, sea vessels, submarine equipment, ports, pipelines, and tanks.
Types of cathodic protection systems
There are two main types of cathodic protection systems: galvanic system and impressed current system. Note that both types include an anode (from which current flows into the electrolyte), a continuous electrolyte from the anode to the protected structure, and an external metal connection.
• Galvanic system:
Galvanic cathodic protection system uses corrosion potentials for various metals. Without cathodic protection, there is one area of the structure with more negative potential than other areas and corrosion occurs. In this case, if an object with a higher negative potential (such as a magnesium anode) is protected near the structure (such as a pipeline) and a metal connection is made, the object becomes an anode and the whole structure becomes a cathode.
Therefore, the galvanic cathode protection system is called a sacrificial anode cathode protection system because the anode is sacrificed to protect the structure. Sacrificial anodes are usually made of metals such as magnesium, zinc and aluminum.
•Impressed current system
Impressed current cathodic protection system is a type of system that is usually applied in cases where there is a high flow requirements to protect against corrosion. The main difference between impressed current systems and sacrificial anodes is that the sacrificial anode system relies on the potential difference between the anode and the structure, while the impressed current system uses an external energy source to conduct the current. Impressed current anodes are usually high silicon cast iron anodes or mixed metal oxide anodes.
- Published in Articles, Educational, Scientific
What is the corrosion?
How does corrosion occur?
Corrosion is an electrochemical process in which current exits a structure at the anode site, passes through an electrolyte, and re-enters the structure at the cathode site. For example, a small section of a pipeline may be anodic because it is located in low-strength soil compared to other pipelines. The current can leave the pipeline at that anode location, pass through the soil, and re-enter the pipeline at the cathodic site. The current flows due to the potential difference between the anode and the cathode. That is, the anode potential is much more negative than the cathode potential, and this difference is the driving force of the corrosion current. The whole system – the anode, the cathode, the electrolyte and the metal connection between the anode and the cathode – is called the corrosion cell.
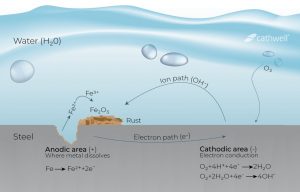
Corrosion Cell
Corrosion consequences
Corrosion is a dangerous and very costly problem and has serious economic, health, safety, technological and cultural consequences for our society. One of the most important problems is the corrosion of structures, which can lead to severe injuries or even loss of life. Safety is compromised by corrosion and damage to bridges, airplanes, vessels, oil and gas pipelines, offshore structures, the entire set of metal structures, and the devices that make up the modern world.

Sample corrosion of pipes
What are the types of corrosion?
There are many different types of corrosion and extensive research is ongoing. Here are some common types of corrosion:
- Uniform corrosion: The most common type of corrosion is uniform reddish corrosion that is evenly distributed throughout the exposed part of the bed.
To prevent uniform corrosion, it is important to ensure proper dewatering and ventilation during design. Also, prevent uniform corrosion from the beginning by constantly cleaning the surfaces and protecting the fasteners with a coating. - Galvanic corrosion: The most common and effective form of corrosion. Occurs when two different metals are in contact in the presence of an electrolyte. In a galvanic cell, the more active metal (anode) is corroded and the more noble metal (cathode) is protected. Numerous factors such as types of metals, relative anode size and environmental conditions (temperature, humidity, salinity, etc.) affect galvanic corrosion.
- Gap corrosion: Gap corrosion occurs in these areas because small cracks tend to be damp and poorly ventilated. The risk of corrosion in the gap multiplies despite the greater number of joints. Slit corrosion is prevented as much as possible by minimizing the use of washers and creating joint connections.
- Published in Articles, Educational, Scientific





