Cathodic protection of pipelines
The role of cathodic protection of pipelines in the oil and gas industry
One of the main and very costly problems in large industries such as gas, oil, petrochemical, shipping, etc., is the corrosion of metal structures.
In the oil and gas industry, carbon steel pipelines are used to transport hydrocarbons from jackets to production platforms and storage tanks, and enter refineries through pipelines. Carbon steel pipelines are the safest and least expensive way to transport large volumes of hydrocarbons over long distances.
Fuel and water transmission pipelines and other structures buried in soil or submerged in water, due to their metallurgical conditions, are in a state of corrosion and after a while, the performance of the structure is disrupted.
Corrosion in the oil and gas industry dramatically increases the overhead costs of plumbing operations due to the replacement of worn equipment and damage to adjacent equipment. Therefore, it is necessary to use a proper protection system to prevent corrosion.
Corrosion control mechanism in cathodic protection of pipelines
Corrosion control mechanisms in oil and gas pipelines are mitigation measures to eliminate or reduce corrosion to prevent pipeline failure and related consequences such as crop failure, environmental pollution and potential accidents.
Five control mechanisms to minimize pipeline corrosion and cathodic protection of pipelines that must be considered during the design and construction of pipelines include design, material selection, coating, cathodic protection, and chemical protection.
Designing
One of the important factors in corrosion control is design. Proper design of a steel pipe increases the life of the structure. Gaps, steel geometry, sharp edges, welded joints, and pipeline connections such as flanges, valves, and pipe supports are often subject to local corrosion due to fluid penetration and dissolution.
In addition, these environments have poor coverage due to inaccessibility or sensitivity to coverage failure. These factors contribute to corrosion and should be carefully considered during the design phase to limit corrosion.
Material selection
The choice of materials is very basic in terms of engineering design to reduce corrosion. The choice of material depends on the environmental conditions such as the fluid being transferred, the temperature, the pressure and the electrolyte in which the pipe is placed (water or soil). Also in flange joints, piping and general joints where it is possible to pair metals of different materials, to prevent galvanic corrosion, care must be taken in determining the material.
Due to its good mechanical properties, availability and low cost, carbon steel, despite its low corrosion resistance compared to other corrosion-resistant alloys, is the main material for pipeline transmission.
coating
One of the main and simplest methods of protecting pipelines is coating. A suitable coating prevents direct contact of the outer surface of the tube with the surrounding environment and thus prevents the flow of ions through the electrolyte, thus limiting corrosion.
There are different methods and types of coating and the choice of its type depends on the type of material selected:
- Painting
Painting is used in environments where the equipment is only exposed to air and no electrolyte is available.
- Coating
The main types of coatings for coating structures that are exposed to electrolytes are strong coatings such as polyethylenes, bitumen and polypropylene.
- Lining
Lining the surface of the structure with rubber sheets.
- Cladding
coating one metal with another.
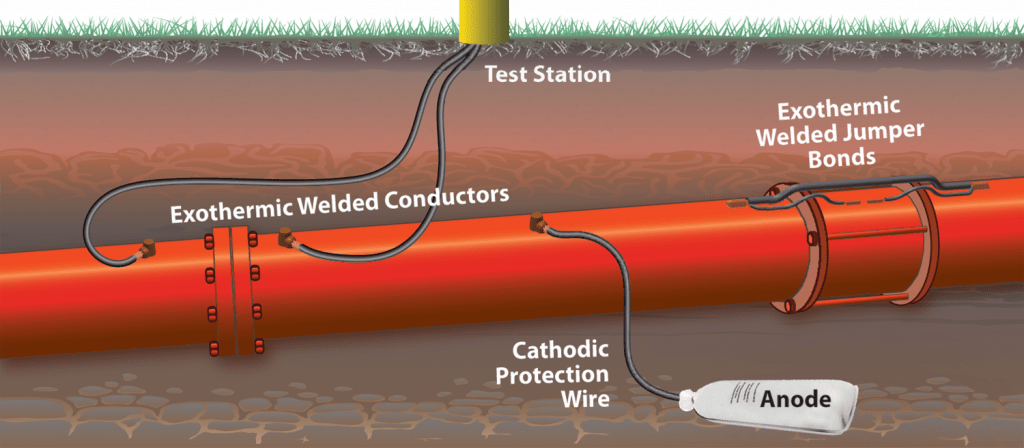
- Cathodic Protection
Using a cathodic protection system along with coating is an effective way to prevent corrosion of pipelines.
There are two general methods for cathodic protection of pipelines:
- Sacrificial anode system
- Impressed current anode system
In cases where the length of the pipeline is very long and in the presence of stray currents, an impressed current system is used to protect the pipeline.
Chemical protection
Chemical protection includes corrosion inhibitors, Oxygen purification system, and biocide in a chemical process.
In the chemical process, biocide is injected into the system, liquid or water to kill microorganisms such as reduced bacteria that increase corrosion under microbiological effects with sulfide. Oxygen scavenging chemicals are injected into the water to deoxygenate to reduce the severity of the corrosion and ultimately achieve adequate cathodic protection of the pipelines.
- Published in Articles, Educational, Scientific
Types of sacrificial anode system backfills and its applications
Types of backfill and its applications:
In cathodic protection systems of pipelines or underground structures buried in the soil, the anode used is not in direct contact with the soil. This is because the minerals and chemicals in the soil may interfere with the conductivity of the stream by creating a high-strength layer. To increase the efficiency of the anode, sometimes magnesium and zinc sacrificial anodes are provided in linen bags with a backfill. The backfill material quickly absorbs soil moisture and reduces electrolyte resistance, thus improving the practical implementation of protection. Backfill materials generally include gypsum plaster, bentonite and sodium sulfate. Depending on the environment in which the anode is placed, one of the types of backfill, which is considered in the table below, is used.
Table of combinations of different types of backfills according to IPS-M-TP-750 standard:
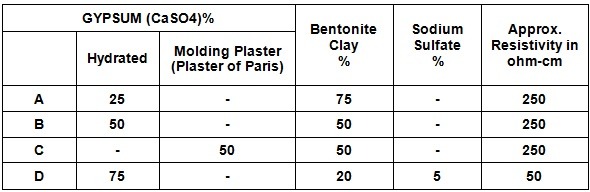
Due to its moisture retention properties, bentonite type A is used in areas where soil moisture is low.
Type B backfill is commonly used for sacrificial zinc anodes.
Type C backfill is useful for magnesium or zinc sacrificial anode in very wet or swampy soils to prevent the backfill from leaving the anode surface quickly.
Type D backfill, this type of backfill has low resistance and is used in areas where soil resistance is high to reduce the resistance of the anode to ground.
Dimensions of backfill bag
According to IPS-M-TP-750 standard, the diameter of the backfill bag must be at least 50 mm larger than the diameter of the anode. The backfill material should be tightly packed in the bag to prevent the anode from moving and to get enough coverage around the anode.

- Published in Articles, Educational, Scientific
What is a sacrificial anode?
How does a sacrificial anode protect metal structures?
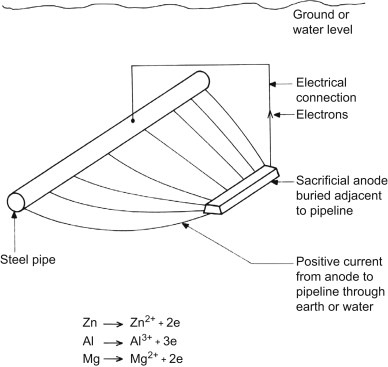
Corrosion of metal structures immersed in water or buried in soil will cause very dangerous and costly damage. One way to protect these structures is to use sacrificial anodes. The sacrificial anode sacrifices itself due to the electrochemical process of corrosion by destroying instead of the impeller, impeller branch, motor or other metal components in water or soil.
Sacrificial anodes are relatively inexpensive metal parts. Known as active metals, they are specifically designed to corrode structures instead of expensive metal parts. These anodes are made of metals that have a more negative voltage than the structural metal. Sacrificial anodes were usually made of zinc metal, hence they are also called “zinc anodes“, but magnesium or aluminum alloy can also be used to produce them. Each of these metals has different properties and applications. The electrical potential and flow capacity of anodic metals are two important features in determining the type and extent of sacrificial anodes used. Sacrificial anodes come in all shapes and sizes, but they all work the same way. They are electrically attached to the metal structure to protect it from corrosion.
How does the sacrificial anode work?
The potential difference between the sacrificial anode and the protected structure causes a current in the electrolyte to flow from the anode to the cathode. The anode and cathode must both be inside an electrolyte and have an electrical connection between them. The sacrificial anode is a metal that has a more negative potential than the structure and forms the negative pole of a corrosion cell. The positive pole of this corrosion cell is the metal (cathode) that protects it. The saltier or more polluted the water, the faster the sacrificial anode will erode.
Since the output current of the sacrificial anodes is much lower than that of the impressed current anodes, these anodes are used in places where a small current is required for protection. Also, in places such as submarine pipelines where we do not have access to electricity and current regulation, the sacrificial anode system is used.
- Published in Articles, Educational, Scientific
What is the cathodic protection?
Cathodic protection and its different types
Cathodic protection is a way to reduce corrosion by minimizing the potential difference between the anode and the cathode. This is achieved by creating a stream from an external source on the structure to be protected (such as a pipeline). When enough current is used, the whole structure will be at a potential. Therefore, there will be no anode and cathode sites. Cathodic protection is commonly used to protect numerous structures against corrosion, such as ships, sea vessels, submarine equipment, ports, pipelines, and tanks.
Types of cathodic protection systems
There are two main types of cathodic protection systems: galvanic system and impressed current system. Note that both types include an anode (from which current flows into the electrolyte), a continuous electrolyte from the anode to the protected structure, and an external metal connection.
• Galvanic system:
Galvanic cathodic protection system uses corrosion potentials for various metals. Without cathodic protection, there is one area of the structure with more negative potential than other areas and corrosion occurs. In this case, if an object with a higher negative potential (such as a magnesium anode) is protected near the structure (such as a pipeline) and a metal connection is made, the object becomes an anode and the whole structure becomes a cathode.
Therefore, the galvanic cathode protection system is called a sacrificial anode cathode protection system because the anode is sacrificed to protect the structure. Sacrificial anodes are usually made of metals such as magnesium, zinc and aluminum.
•Impressed current system
Impressed current cathodic protection system is a type of system that is usually applied in cases where there is a high flow requirements to protect against corrosion. The main difference between impressed current systems and sacrificial anodes is that the sacrificial anode system relies on the potential difference between the anode and the structure, while the impressed current system uses an external energy source to conduct the current. Impressed current anodes are usually high silicon cast iron anodes or mixed metal oxide anodes.
- Published in Articles, Educational, Scientific
Tavanazob Sana’ati Kavir Company is the sponsor of the 19th National Corrosion Congress
Informs professors, researchers, entrepreneurs, craftsmen, and students related to corrosion science and engineering and all interested parties that the “19th National Corrosion Congress” will be held on March 26 and 27, 2009 in Tehran.
Although corrosion and its problems are more than 110 years old in the Iranian oil industry, but dealing with the phenomenon of corrosion in university programs, research centers and other industries in Iran began about 50 years ago. Therefore, due to the importance of the issue and the loss of life, environment, and finance resulting from this phenomenon, scientific, technical and economic studies and research and educational activities on corrosion since 1348 were organized and implemented in the country.
Initially, congresses, seminars and gatherings were organized in a decentralized manner in the country’s academic and industrial centers; Until in 1986, a codified plan was made to hold national corrosion congresses on a regular and biennial basis, followed by the first congress in 1988, the second in 1990 and the third in 1993.
After the establishment of the Iranian Corrosion Association and obtaining official permission from the Ministry of Science, Research and Technology in December 1993, subsequent congresses have been held by the Iranian Corrosion Association in cooperation with universities, research centers, and industries nationally or internationally.
On the eve of the 19th National Corrosion Congress, the Iranian Corrosion Association invites professors, researchers, craftsmen, legal and real members of the Iranian Corrosion Association, and all interested engineers and students to participate and get acquainted with the axes of the Congress and the process of submitting valuable articles and research. , Refer to the association’s website at congress.ica.ir.
- Published in Educational, Research, Scientific







