What is the corrosion?
How does corrosion occur?
Corrosion is an electrochemical process in which current exits a structure at the anode site, passes through an electrolyte, and re-enters the structure at the cathode site. For example, a small section of a pipeline may be anodic because it is located in low-strength soil compared to other pipelines. The current can leave the pipeline at that anode location, pass through the soil, and re-enter the pipeline at the cathodic site. The current flows due to the potential difference between the anode and the cathode. That is, the anode potential is much more negative than the cathode potential, and this difference is the driving force of the corrosion current. The whole system – the anode, the cathode, the electrolyte and the metal connection between the anode and the cathode – is called the corrosion cell.
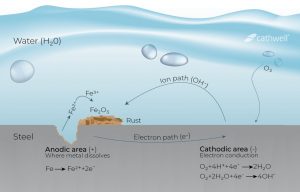
Corrosion Cell
Corrosion consequences
Corrosion is a dangerous and very costly problem and has serious economic, health, safety, technological and cultural consequences for our society. One of the most important problems is the corrosion of structures, which can lead to severe injuries or even loss of life. Safety is compromised by corrosion and damage to bridges, airplanes, vessels, oil and gas pipelines, offshore structures, the entire set of metal structures, and the devices that make up the modern world.

Sample corrosion of pipes
What are the types of corrosion?
There are many different types of corrosion and extensive research is ongoing. Here are some common types of corrosion:
- Uniform corrosion: The most common type of corrosion is uniform reddish corrosion that is evenly distributed throughout the exposed part of the bed.
To prevent uniform corrosion, it is important to ensure proper dewatering and ventilation during design. Also, prevent uniform corrosion from the beginning by constantly cleaning the surfaces and protecting the fasteners with a coating. - Galvanic corrosion: The most common and effective form of corrosion. Occurs when two different metals are in contact in the presence of an electrolyte. In a galvanic cell, the more active metal (anode) is corroded and the more noble metal (cathode) is protected. Numerous factors such as types of metals, relative anode size and environmental conditions (temperature, humidity, salinity, etc.) affect galvanic corrosion.
- Gap corrosion: Gap corrosion occurs in these areas because small cracks tend to be damp and poorly ventilated. The risk of corrosion in the gap multiplies despite the greater number of joints. Slit corrosion is prevented as much as possible by minimizing the use of washers and creating joint connections.
- Published in Articles, Educational, Scientific


